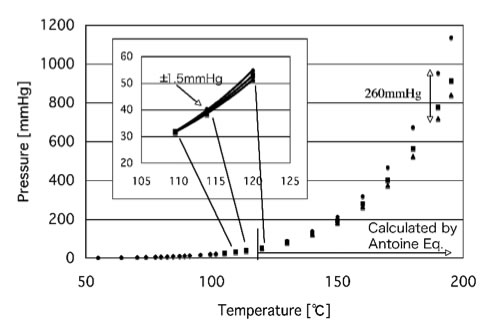
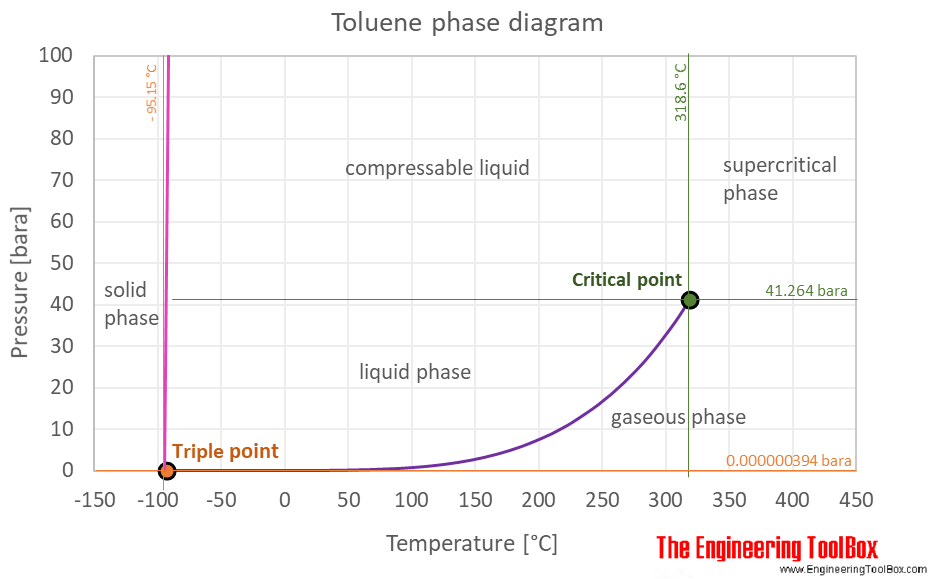
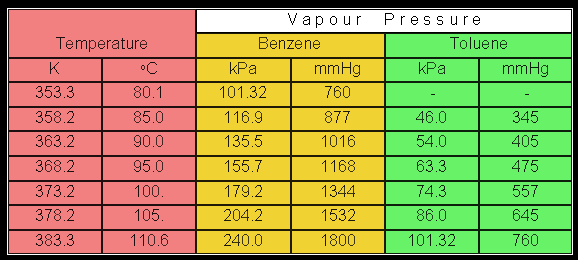
Boiling points of crude oil solution {crude oil diluted with toluene... | Download Scientific Diagram

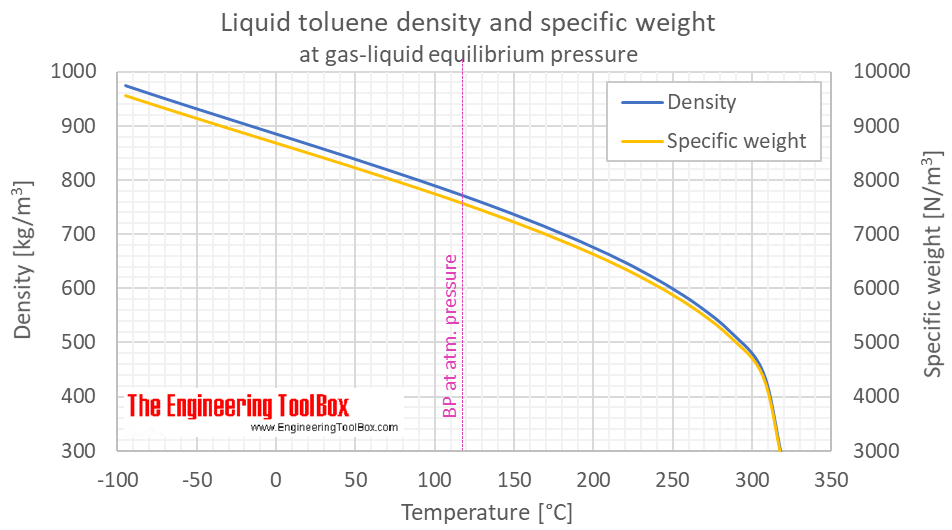
The boiling point elevation constant for toluene is \( 3.32 \mathrm{~K} \) \( \mathrm{kg} \mathr... - YouTube
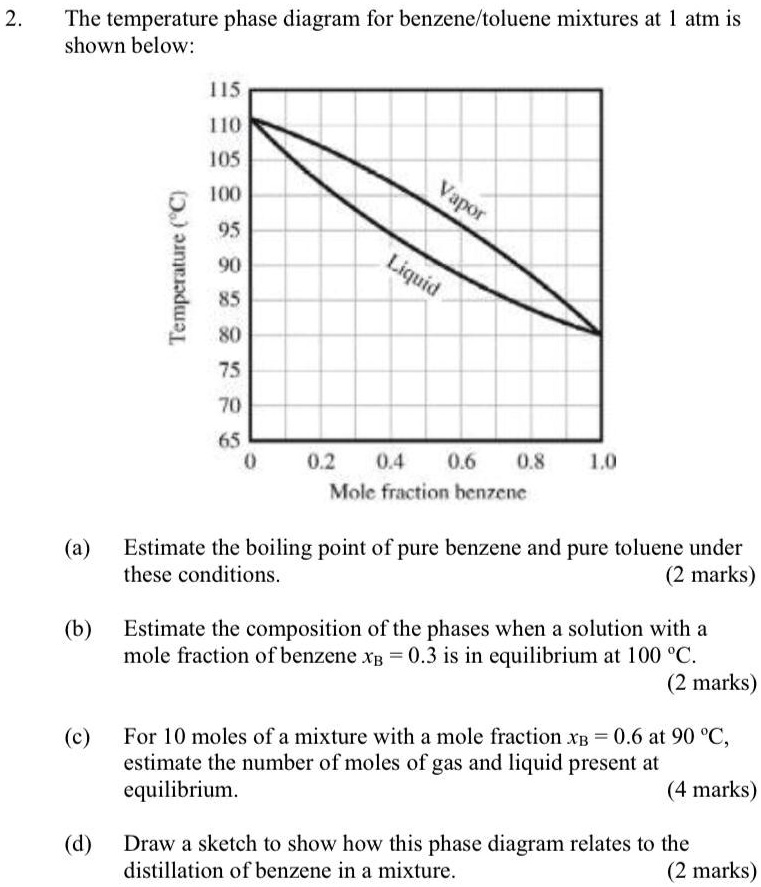
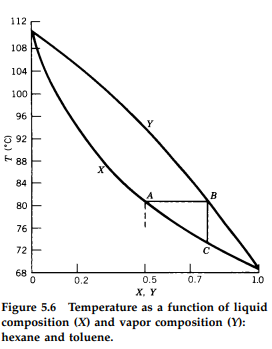
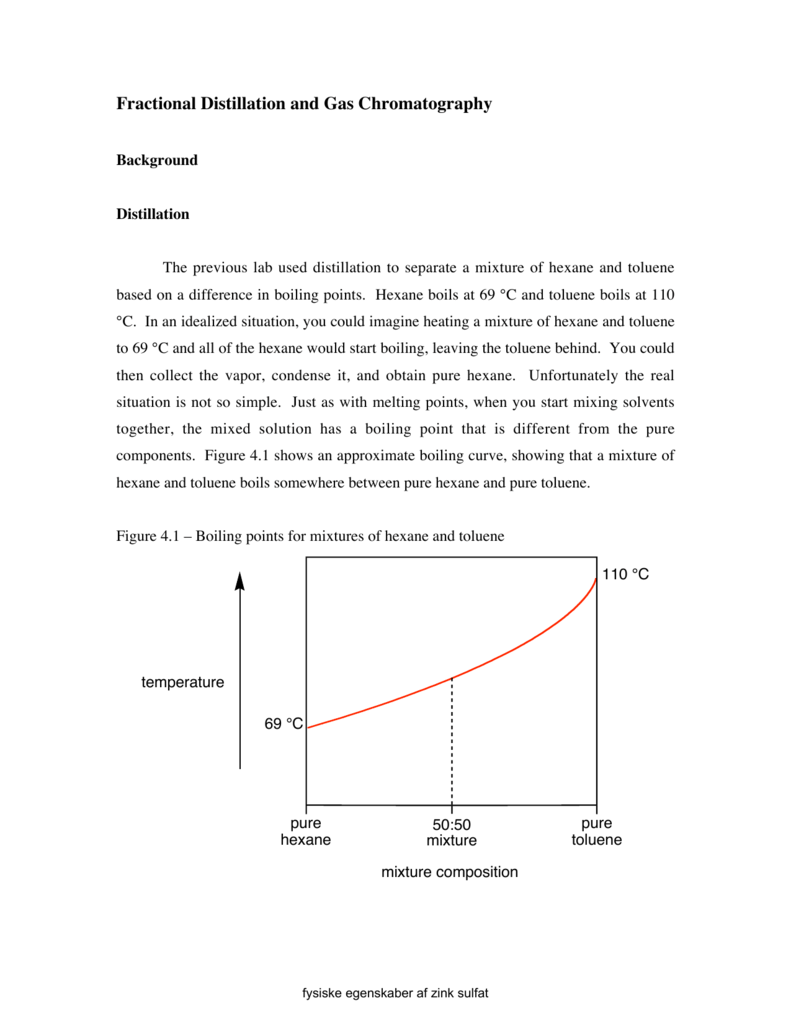
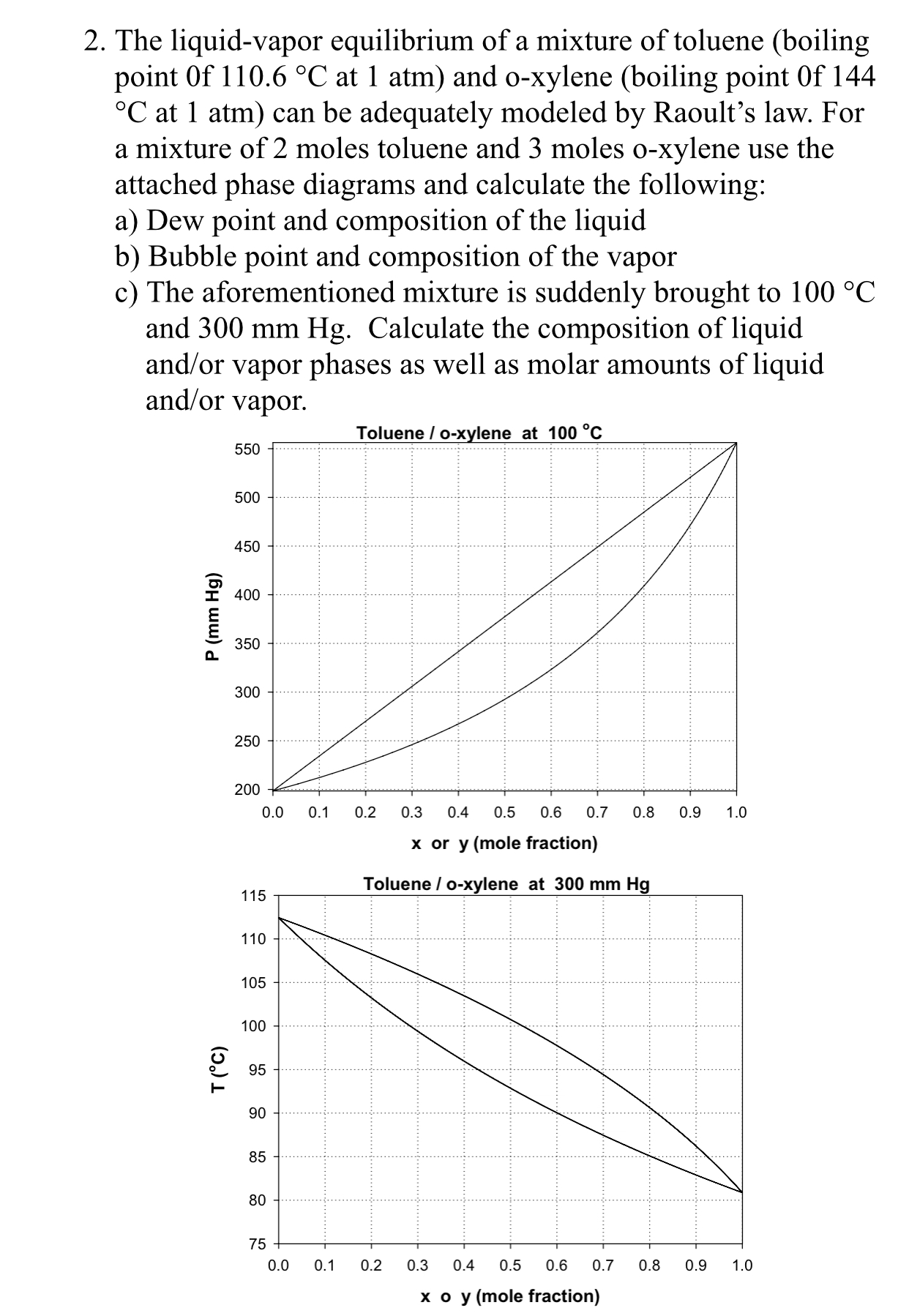
SOLVED: The temperature phase diagram for benzene/toluene mixtures at 1 atm is shown below: H5 MU 105 0 16 95 Yu L 85 Xu 75 70 65 02 04 0.6 08 Molc

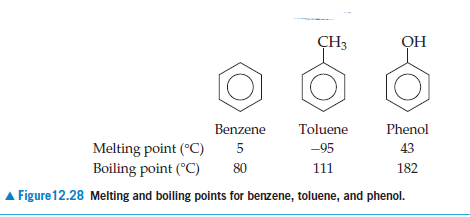
The normal boiling point of toluene is 110.7^0C and its boiling point elevation constant is 3.32K kg mo1^-1 . The enthalpy of vapourization of toluene is nearly:

Boiling point T b ,°С, vs. molecular weight M: (1): (a) benzene, (b)... | Download Scientific Diagram

The normal boiling point of toluene is 110.7^0C and its boiling point elevation constant is 3.32K kg mo1^-1 . The enthalpy of vapourization of toluene is nearly:
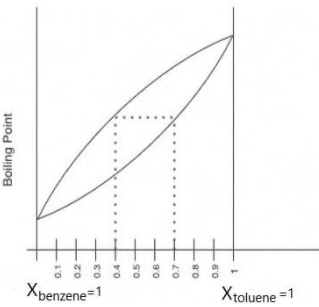
The following question represents the variation of boiling point with the composition of liquid and vapours of binary liquid mixtures. The graph is plotted at constant pressure. Which of the following statement(s)
Toluene, C6H5CH3, is a liquid used in the manufacture of TNT. Its normal boiling point is 111.0°C, and its molar heat of vaporization is 35.9 kJ/mol. What would be the vapor pressure (
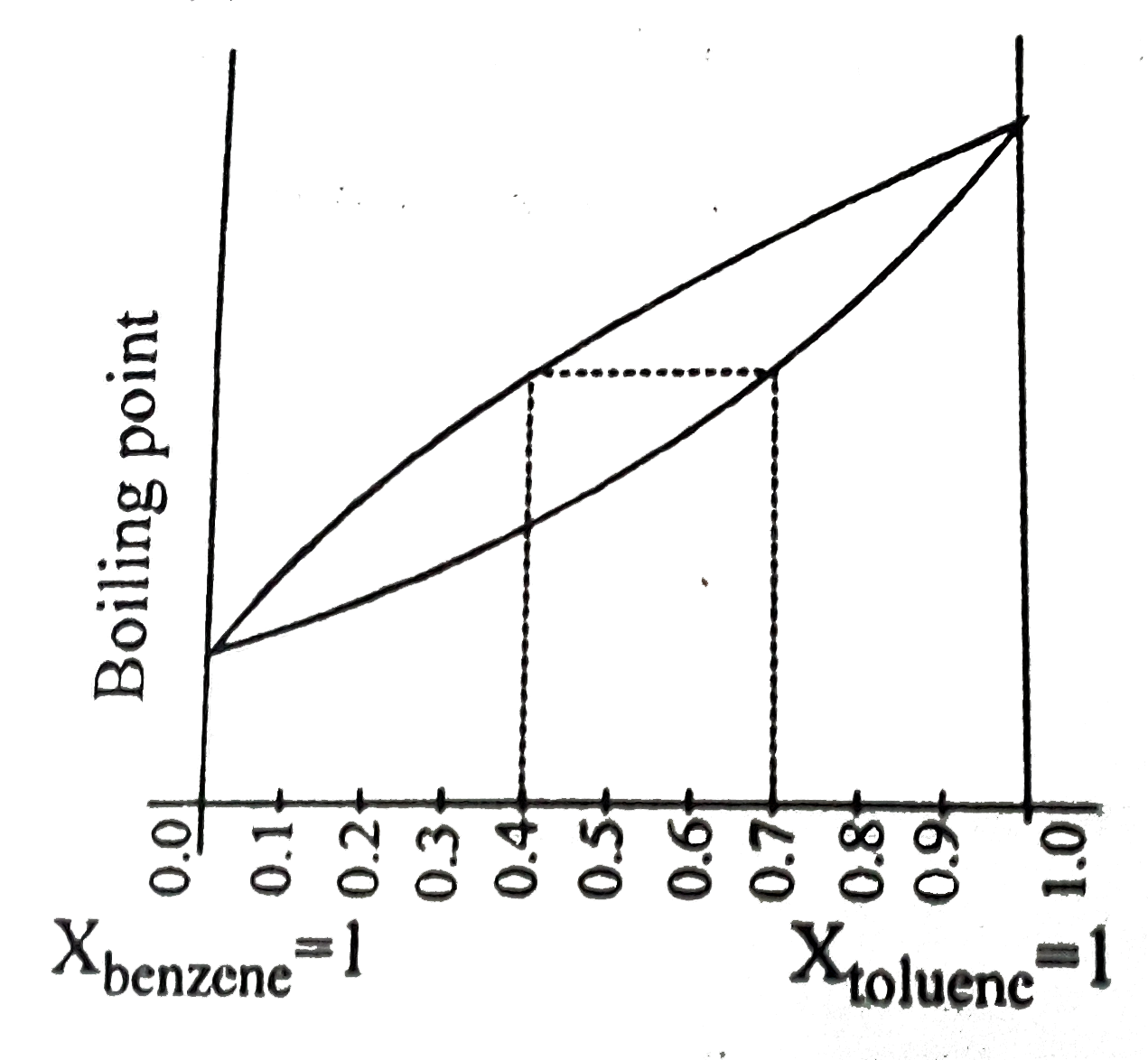
![1. The boiling point diagram for a mixture of benzene and toluene is shown below. [{Image src='fraction7304253463166023866.jpg' alt='fraction' caption=''}] a. What is the boiling point of pure toluen | Homework.Study.com 1. The boiling point diagram for a mixture of benzene and toluene is shown below. [{Image src='fraction7304253463166023866.jpg' alt='fraction' caption=''}] a. What is the boiling point of pure toluen | Homework.Study.com](https://homework.study.com/cimages/multimages/16/fraction7304253463166023866.jpg)